Lighting for human-occupied space requires a spectral optimization balancing act between four competing objectives:
- Illumination — Light that provides human color vision with the discrimination needed to perform tasks, accurately renders color, and is aesthetically pleasing
- Human health — Light that robustly synchronizes the circadian timing system during the day and prevents the harmful circadian disruptive health effects of light at night
- Human productivity — Light that stimulates human alertness, cognitive performance, and mood to optimize productivity and creativity, while promoting good quality sleep at night
- Energy efficiency — Light sources that consume the fewest watts to achieve all the aforementioned objectives
The most common failure of modern electric lighting is that it is optimized to meet only one or two of these critical objectives while undermining other equally important functions. For example, most of today’s mass-produced LEDs are optimized to achieve energy efficiency at the cost of impairing human health and productivity, and in some cases providing aesthetically unattractive lighting. This leaves lighting specifiers and end users with an unacceptable choice: Do I select the most energy-efficient LED at the expense of human health and performance?
Today’s commonly used lighting metrics also do not consider human health. For example, the energy-efficiency metric of lumens per watt (lm/W) only measures the electrical energy efficiency of meeting the CIE 1924 photopic illumination objective, not the electrical efficiency of meeting circadian or human productivity objectives. And correlated color temperature (CCT), while useful in aesthetic design, is a misleading guide to human health and productivity optimization.
Spectrally optimized LEDs are the future of lighting
The precision spectral engineering made possible by LED-based solid-state lighting (SSL) technology opens the door to producing light that meets all four key objectives. Because each of these objectives has a unique spectral sensitivity within the visible light spectrum (380–780 nm) and an effective threshold level required for a desirable outcome, we can engineer an optimization balancing act between these competing objectives.
In this article, we will examine in turn the unique spectral sensitivity and the effective threshold levels of each of the four fundamental competing objectives — illumination, health, productivity, and energy efficiency — before we examine overall lighting optimization solutions.
To be clear, optimization is not the same as correlation. A range of lighting spectral solutions may be statistically correlated with a desirable outcome (e.g., reduced circadian disruption). In contrast, the optimized outcome will be the spectral solution that maximizes the desirable outcome (e.g., achieves minimal circadian disruption). Because we must balance competing objectives when designing lighting, it is critical to know the optimized spectral sensitivity and the effective threshold levels of each objective before starting to make tradeoffs.
Illumination optimization
Illumination requires balancing the light spectrum needed to optimize visual discrimination while providing a mix of wavelengths in white light that will enable all colors to be accurately perceived. Optimal human visual discrimination in normal well-lit conditions is determined by the averaged response of the three main types of color vision cones, described by the photopic luminosity function. The peak of this luminosity function is at 555 nm because the human eye’s color-vision image forming system is most sensitive to green light at this wavelength.
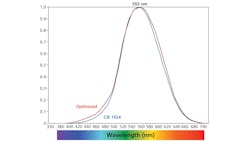
The standard illumination metrics such as lumens, lux, candela, and footcandles are calculated by weighting the irradiant power contribution of each visible wavelength (380–780 nm) according to the outdated CIE 1924 version of luminosity function (Fig. 1, blue line). More recent accurate measurements of luminosity (Fig. 1, red) show that the standard photopic curve underestimates the effect of blue and violet wavelengths by approximately 50%.1 This explains why blue- and violet-pump LEDs appear brighter to the human eye than is indicated by light-meter lux.
So, in the SSL era, it would be prudent to use the corrected photopic function for lighting optimization. However, even this corrected photopic function, and colorimetric values such as CCT and CRI, tell us virtually nothing about the human health or human productivity effectiveness, or the energy efficiency of the light in meeting all the key objectives.
Effective illumination thresholds
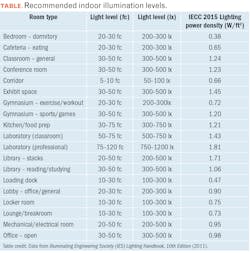
For many years, standard tables have been published indicating the recommended desktop horizontal illumination levels depending on the type of task being performed, the extent to which the surrounding surfaces reflect or absorb light, and the visual acuity of the individual.2 As shown in the nearby table, minimum threshold levels of 200–500 lx are required for most visual tasks.
Human health optimization
Over the past 20 years, too much blue-rich light exposure at night, and too little blue-rich light during the day, has been linked to dozens of serious health disorders caused by circadian rhythm disruption, including sleep disorders, depression, obesity, diabetes, cardiovascular disease, and several hormone sensitive cancers including breast and prostate cancer.3-5
These effects should be distinguished from the so-called “blue light hazard” of short-wavelength light, which requires light intensities never seen in indoor lighting. The retina damage effects of so-called “bad blue light” are only seen at bright sunlight levels of light intensity. So in illuminating occupied space, we only need to be concerned with the circadian effects of blue light.
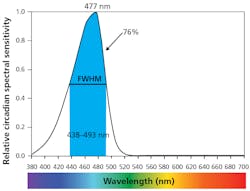
Circadian spectral optimization. The circadian effects are mediated by blue-sensitive photoreceptors in the eye called intrinsically photosensitive retinal ganglion cells (ipRGCs) and are communicated to the suprachiasmatic nucleus (SCN) — the biological clock that regulates our circadian rhythms. Under normal workplace lighting conditions (approximately 500-lx desktop horizontal illumination, 12-hour work shift), the human circadian potency spectral sensitivity curve has a peak at 477 nm with a full-width half-maximum of 438–493 nm “circadian blue” wavelengths, which accounts for 76% of the circadian potency of light.6 This narrow band of blue light synchronizes our circadian rhythms during the day but can disrupt them at night.
Optimization of circadian lighting models. Previous published circadian sensitivity curves were either based on short (30–90 minute) exposures of people under dark-adapted conditions with pharmacologically dilated pupils to mostly monochromatic (single-color) lights with peak sensitivity between 465–485 nm (Circadian Stimulus [CS], Circadian Active Factor), or were based on a 1-minute cellular calcium response to camera flashes of light onto human melanopsin photopigment in tissue culture with a peak sensitivity at 479 nm (Equivalent Melanopic Lux [EML]). In contrast, the circadian potency curve in Fig. 2 was derived from people living for extended periods under real-world workplace lighting conditions.6 (See https://vimeo.com/428559469 for more detail.)
The EML circadian model and the CIE 2018 standard were refined by right-shifting this 479-nm melanopsin sensitivity curve by 11 nm to derive a Melanopic Equivalent Daylight Illuminance (MEDI) with a peak sensitivity at 490 nm.7 But there is no evidence that this theoretical correction for pre-receptorial processing results in the optimized circadian sensitivity curve. To be sure, statistical correlations of physiological data with the MEDI function have been reported, but merely showing an acceptable R2 correlation to data is not the same as finding the optimal R2 correlation.
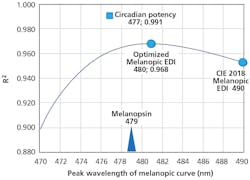
To illustrate this, we conducted an optimization of peak wavelength sensitivity (λmax) for the MEDI function (Fig. 3) by progressively shifting the melanopic curve λmax in 1-nm increments from 470 to 490 nm, and testing the correlation with the total overnight melatonin measured in 34 people working 12-hour overnight shifts under seven spectrally-diverse light sources.
What was striking was that all λmax values for MEDI between 470–490 nm had acceptable R2 correlations in excess of 0.9. However, the optimal melanopic curve fit with an R2 of 0.968 was at λmax = 480.8 nm, suggesting that the pre-receptorial adjustment was not justified, or was compensated for by post-receptorial SCN or pineal processing. In comparison, the circadian potency curve fit in Fig. 3 did not assume the melanopic function and allowed for a skewed sensitivity curve, and therefore had a better fit (R2 = 0.991 at 477 nm) to the overnight human melatonin data.
In contrast, we found no correlation whatsoever (R2 = 0.001) between the CS values as described in UL DG 24480,8 and the overnight human melatonin data from our 34 subjects under normal workplace lighting conditions.6 It was remarkable that even very diverse spectra such as the Day and Night SPDs in Fig. 6 (bottom) with very different circadian effects have the almost the same CS value (0.30 day, 0.29 night) at 300 lx of photopic illumination.
Effective circadian blue thresholds. Unlike illumination, which is measured horizontally at the desktop, circadian-effective light is measured as vertical irradiance of circadian blue entering the eye (corneal blue irradiance in µW/cm2). Under natural outdoor lighting conditions, our circadian rhythms are kept robustly synchronized, and our sleep-wake cycles and body functions healthy, by the considerable contrast in corneal circadian blue levels between day and night.
As Fig. 4 shows, the blue corneal irradiance at night even under the brightest moonlight (0.02 µW/cm2) is several thousand-fold less than the blue corneal irradiance during the normal day whether it is overcast and rainy (60 µW/cm2) or looking south with a bright blue sky (3500 µW/cm2). In the pre-electric era, natural outdoor daylight (excluding twilight and night) delivered to our eyes blue corneal irradiance levels ranging from about 20 µW/cm2 on the darkest day to 7000 µW/cm2 on the brightest sunny day.
The sensitivity of human circadian clocks to circadian blue-light wavelengths can be measured using a key marker of the circadian timing system — the level of melatonin hormone released at night.9 Minimal changes in melatonin suppression occur from the lowest levels of nocturnal light up to a blue corneal irradiance of about 2 µW/cm2. But as levels of circadian blue light increase above 2 µW/cm2, the impact on melatonin levels rapidly grows, reaching 50% suppression at 20 µW/cm2. Thereafter, over the natural range of daytime illumination (20–7000 µW/cm2) the rate of change slows, reaching 70% suppression at sunny day blue corneal irradiance levels of 1000–7000 µW/cm2.
This suggests that the threshold daytime dosage levels of circadian blue required to ensure circadian rhythms are robustly synchronized is approximately 20 µW/cm2, and the minimum level of circadian blue that can safely be used at night without causing circadian disruption is approximately 2 µW/cm2. These threshold levels of <2 µW/cm2 for evening and nighttime lighting (sunset to sunrise), and >20 µW/cm2 for daytime lighting (sunrise to sunset) have been confirmed by examining multiple studies of circadian lighting where the effects on sleep, circadian rhythms, clock genes, cognitive performance, melatonin suppression, and other health metrics have been evaluated.10
Corneal blue irradiance can be easily measured without any complex model or calculator, by using a spectrometer held at eye level pointed in the field of gaze and then summing the values of corneal blue irradiance from 438–493 nm.
Furthermore, light sources can be easily specified using a convenient rule of thumb. Lights that emit >20% of their total visible irradiance (380–780 nm) as 438–493 nm circadian blue will deliver the required daytime level of >20 µW/cm2 in typical room lighting conditions of 300–500 lx desktop illumination. And lights that emit <2% of their total visible irradiance (380–780 nm) as 438–493 nm circadian blue will deliver the required nighttime level of <2 µW/cm2 in corneal blue irradiance in typical room lighting conditions of 300–500 lx desktop illumination. UL has issued a verification mark for light fixtures that emit <2% blue at night.
Human productivity optimization
Light also has an acute stimulatory effect on the central nervous system, which boosts alertness, cognitive performance, and mood, the essential ingredients of human productivity and creativity. It can be more effective than a cup of coffee. The alerting effect progresses from alertness-related subcortical structures (hypothalamus, brainstem, thalamus) and limbic areas (amygdala and hippocampus) to cortical regions associated with the ongoing task, ultimately affecting behavior and productivity. It is a direct effect lasting if the light is on but fades quickly once the light is switched off.
Spectral sensitivity. Shorter violet and blue wavelengths (420–480nm) have been shown to be more effective in stimulating alertness than longer green and yellow wavelengths (550–600 nm). The peak optimal effects appear to be in the violet range (about 420 nm).11 Thus, this direct alerting effect of light has a very different optimal wavelength than photopic illumination or circadian potency. This means it is possible to spectrally engineer light for evening and nighttime illumination with a violet LED pump, which enhances alertness and cognitive performance without adversely impacting circadian rhythms. Then during the day, LEDs that emit the circadian blue wavelengths required for entrainment of the circadian system have the added advantage of also providing alertness stimulation.
Threshold. A greater enhancement in alertness is seen with 11 µW/cm2 of 420-nm corneal violet light than with 26 µW/cm2 of 470-nm corneal blue light (Fig. 6). Other studies have shown enhanced alertness with 12 µW/cm2 of 460-nm corneal blue light. Thus, the level of corneal circadian blue irradiance that enhances cognitive performance and reduces attentional lapse is comparable to the >20 µW/cm2 required for robust circadian synchronization.
But using these levels of blue light for enhancing alertness and performance after sunset is not desirable, because it significantly exceeds the 2-µW/cm2 threshold for causing circadian disruption. Fortunately, it is possible to use 11-µW/cm2 violet light instead of blue light during nocturnal hours, as that does not disrupt the circadian system, and still provides the desired alerting effect. Then during the day, the blue pump LEDs, which provide the required >20-µW/cm2 circadian blue light for robust circadian entrainment, will also provide enough blue light to ensure optimal alertness and cognitive performance.
Energy efficiency optimization
At the Hotel Gat near Checkpoint Charlie in Berlin, Germany you can stay in a room lit by monochromatic green light12 near the 555-nm peak of the photopic luminosity curve. This lighting comes close to meeting the maximum possible energy efficiency of 683 lm/W. But most of us would rather trade a loss of energy efficiency for polychromatic white light that uses a combination of other less energy-efficient wavelengths.
However, lm/W only indicates the energy consumed in providing the photopic illumination by light. It says nothing about the efficacy of providing circadian lighting, or lighting that optimizes human productivity by using shorter wavelengths with little impact on photopic lumens.
A more relevant measure for circadian lighting efficiency would be circadian irradiance/watt, which would measure the energy required to provide the daytime-effective threshold of 20 µW/cm2 of 438–493 nm blue corneal irradiance. And a measure of human productivity lighting efficiency might quantify a variable such as alerting irradiance/watt, which would indicate the electrical energy required to meet the alertness threshold of blue or violet corneal irradiance.
Conclusion
Now that we have defined the optimized spectra and thresholds for photopic illumination, healthy circadian function, and human productivity, we can look at what it takes to derive the optimal balance for lighting that is truly fit for human use.
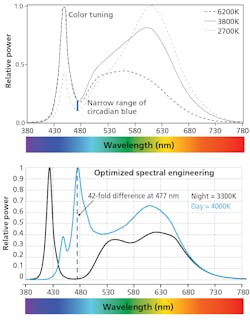
White color tuning where the CCT of approximately 450-nm-pump white LED lights is varied up and down the black body line is one method, albeit a very inefficient one, of delivering more blue-rich light during the day and less blue-rich light at night.
For example, the spectral power distributions (SPDs) of the color-tunable lights used by Pacific Northwest National Laboratory (PNNL) in its recent assessment of energy efficiency of human-centric lighting13 (see also a report by LEDs Magazine) have little difference in relative blue irradiance around the circadian sensitivity peak of 477 nm, despite large changes in CCT from 2700K to 6200K (Fig. 6, top). This means to deliver an effective daytime stimulus at the threshold of >20 µW/cm2 of 438–493-nm blue corneal irradiance would require 4× the power (W) and light intensity than that required to meet the safe night threshold of <2 µW/cm2. Such color-tunable lights are thus an inefficient and unacceptable solution for circadian lighting, even without considering the excessively harsh CCT of 6200K during the day and yellowish 2700K CCT during evening hours.
In contrast when daytime and nighttime LED chips are spectrally engineered to optimize illumination, circadian efficacy, human productivity, and energy efficiency14 (Fig. 6, bottom), a more energy-efficient delivery of circadian lighting is obtained within a narrower 3300–4000K CCT range. With a 42-fold difference in circadian blue irradiance at 477 nm between the day LEDs and night LEDs at the same lux levels, it is possible to deliver the required day >20 µW/cm2 and night <2 µW/cm2 circadian blue irradiance without changing illumination lux levels, or energy consumption, and with minimal changes in CCT. Furthermore, with these spectrally engineered LEDs, the WELL v2 standard can be met with 40% lower desktop photopic lux levels than traditional LEDs.
A further interesting feature of such spectrally engineered circadian lights is that human productivity can be enhanced 24/7 whenever the lights are on using ~420-nm violet light during evening hours and ~477-nm blue light during the day. As the 3-30-300 rule ($3/sf utilities, $30/sf occupancy, and $300/sf workforce costs) reminds us, any energy savings derived by SSL are dwarfed by their impacts on human productivity and health.
The potential of advanced spectral engineering is clear. All lights for human-occupied space should provide high-quality illumination, support human health and productivity, and do it all in an energy-efficient manner. That can only be achieved with precisely defined optimized spectra, and by meeting effectiveness thresholds for each objective so appropriate tradeoffs can be determined.
REFERENCES
- L.T. Sharpe, A. Stockman, W. Jagla, and H. Jagle, “A luminous efficiency function V*(λ) for daylight adaptation,” J. Vision, 5, 948–968 (2005), and correction in Ibid (2011) “A luminous efficiency function, VD65* (λ), for daylight adaptation: A correction,” Col. Res. Appl., 36, 42–46 (2010).
- D.L. DiLaura, W. Houser, R.G. Mistrick, and G.R. Steffy, The Lighting Handbook, 10th Edition, Illuminating Engineering Society (2011).
- K.M. Zielinska-Dabkowska, “Make lighting healthier,” Nature, 553, 7688, 274–276 (2018).
- US National Toxicology Program, Draft Report on Carcinogens Monograph on Night Shift Work and Light at Night, US National Institute for Environmental Health Sciences (2018).
- B. McGroarty, “Focus Shifts from Sleep to True Circadian Health,” 2020 Wellness Trends, Global Wellness Summit (2020).
- M. Moore-Ede, A. Heitmann, and R. Guttkuhn, “Circadian Potency Spectrum with Extended Exposure to Polychromatic White LED Light under Workplace Conditions,” J. Biol. Rhythms, 35, 405–415 (2020).
- CIE International Standard (CIE S 026/E:2018) System for Metrology of Optical Radiation for ipRGC-Influenced Responses to Light, Commission Internationale de L’Eclairage, Central Bureau Vienna, Austria (2018).
- UL DG 24480, Design Guideline for Promoting Circadian Entrainment with Light for Day-Active People (2020).
- K.E. West et al., “Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans,” J. Appl. Physiol., 110, 3, 619-626 (2011).
- M. Moore-Ede, “Day and Night Specifications for Effective Circadian Lighting” (2021).
- V.L. Revell, J. Arendt, L.F. Fogg, and D.J. Skene, “Alerting effects of light are sensitive to very short wavelengths,” Neurosci. Lett., 399, 96–100 (2006).
- TripAdvisor, Hotel Gat Point Charlie review.
- S. Safranek, J.M. Collier, A. Wilkerson, and J.G. Davis, “Energy impact of human health and wellness lighting recommendations for office and classroom applications,” Energy & Buildings, 226, 110365 (2020).
- Circadian ZircLight, “Blue Light InSight: Zirc™ LED Technology.”
Get to know our expert
MARTIN MOORE-EDE, MD, PhD, is founder and CEO of Circadian ZircLight. For more than 30 years, Dr. Moore-Ede has been a leading expert on circadian clocks and their entrainment and disruption by light. As a professor at Harvard Medical School (1975–1998), he led the team that located the suprachiasmatic nucleus (SCN), the biological clock in the human brain that controls the timing of sleep and wake, and pioneered research on how the human body can safely adapt to working around the clock. At Circadian ZircLight, Dr. Moore-Ede leads the development and medical research validation of spectrally-engineered Zirc™ LED DaySync™ and NightSafe™ chips and luminaires. The white-light fixtures precisely time the delivery of circadian blue wavelengths to boost daytime productivity, minimize circadian disruption, and remove the harmful effects of circadian blue light at night.
Enjoyed this article? Visit our digital magazine for more like this >>
![FIG. 4. The melatonin sensitivity curve is correlated to circadian blue and the night-and-day blue corneal irradiance thresholds. Blue corneal irradiance is plotted logarithmically against the degree of melatonin suppression. [Image credit: Data replotted from West et al. (2011) - https://bit.ly/3fb8PoS.] FIG. 4. The melatonin sensitivity curve is correlated to circadian blue and the night-and-day blue corneal irradiance thresholds. Blue corneal irradiance is plotted logarithmically against the degree of melatonin suppression. [Image credit: Data replotted from West et al. (2011) - https://bit.ly/3fb8PoS.]](https://img.ledsmagazine.com/files/base/ebm/leds/image/2021/04/2104LED_moo_4.60789bb25676a.png?auto=format,compress&fit=max&q=45&h=142&height=142&w=250&width=250)
![FIG. 5. Relative sustained alertness over a four-hour period produced by monochromatic lights of different wavelengths is evaluated. Even lower intensities of 420-nm light stimulated more alertness than higher intensities of 470-nm and 600-nm light. ]Image credit: Data replotted from Revell et al. (2006; https://bit.ly/3d25k1d).] FIG. 5. Relative sustained alertness over a four-hour period produced by monochromatic lights of different wavelengths is evaluated. Even lower intensities of 420-nm light stimulated more alertness than higher intensities of 470-nm and 600-nm light. ]Image credit: Data replotted from Revell et al. (2006; https://bit.ly/3d25k1d).]](https://img.ledsmagazine.com/files/base/ebm/leds/image/2021/04/2104LED_moo_5.607db83c64c3a.png?auto=format,compress&fit=max&q=45&h=124&height=124&w=250&width=250)