This is the second article in a two-part series that addresses ultraviolet (UV) and 405-nm lighting technology’s source lifetime, environmental and operational impact, and efficacy. A prior article examined the challenges of UV lighting, argued the need for standards and metrics, and offered a comparison to whole-room disinfection systems. Both articles are based on research described in the paper “The virucidal effects of 405 nm visible light on SARS-CoV-2 and influenza A virus” by Scientific Reports, a Nature publication, on Sept. 30, 2021.1
For years, whole-room disinfection systems have been utilized in healthcare to mitigate the transmission risk of infectious disease. With the coronavirus pandemic, many people managing nonhealthcare environments — everything from schools to cruise ships to commercial offices — are considering deploying these systems. However, commercial buyers and end users typically don’t have the same level of experience with germicidal systems and their considerations (safety, efficacy, source lifetime, and environmental impact) as the healthcare providers who typically install these technologies. I covered safety guidelines and metrics in my first article; this article highlights nonsafety considerations — such as system features, space utilization, surfaces, and occupancy — in a general context, to help potential end users make a more informed decision.
Source lifetime
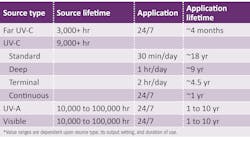
With use, light sources degrade over time. The rate of degradation can be heavily influenced by the output at which the device operates. Therefore, users should identify the expected lifetime for the product being considered under the maximum output conditions described in the manufacturer’s claim. For example, a UV-C source used in a surgical suite may have settings related to standard, deep, and terminal cleaning, with lamp lifetimes of 18 years, 9 years, and 4.5 years, respectively; these would correspond to a daily use of 30 minutes, one hour, and two hours.
Traditional UV-C lamps may have a 9,000-hour source lifetime, while newer far UV-C sources (emitting wavelengths from 200 to 230 nm, according to the International Ultraviolet Association) have advertised a 3,000-hour lifetime. When operated continuously, these sources would need to be replaced approximately every four to twelve months. For reference, UV-A (315 to 400 nm) and visible blue (450 to 495 nm) LEDs can operate from 10,000 to 100,000 hours depending on the output, thermal design, and LED packaging architecture used (summarized in Table 1). Users should contact their manufacturer for L70 (source lifetime) data related to their specific product design and application.
Once the source lifetime is understood, users can evaluate the impact of lifetime on the efficacy and total cost of ownership of the disinfection system. Turning the source off occasionally will extend the time between replacement; however, disinfection will be noncontinuous. In this case, users need to ask the manufacturer for efficacy data based on episodic (noncontinuous) usage. Other relevant questions to ask when considering an environmental disinfection system are:
- How do I know when to change the source?
- How is the source changed?
- How much does the system performance degrade prior to this change?
- How much does replacing the source cost, including parts and labor?
System design factors
Environmental disinfection systems can affect room design, occupants, and materials within the space. Multiple room integration and operational factors need to be addressed as part of the system design and evaluation process.
First, is the disinfecting source integrated with existing lighting or is it an overlay? If it is an overlay, the installation will require additional power, controls, and ceiling mounting hardware, resulting in potentially higher installation costs when compared to the actual product cost.
Can the disinfection function be applied continuously while the room is occupied, or must it be applied episodically while the room is shut down? For rooms that need to be in use 24/7 or at specific times of day, shutting down occupancy can have a financial impact to the operator due to lost revenue, such as closing down a surgical suite. On the other hand, continuous disinfection addresses contamination created by room occupants while they are in the space.
What type of system maintenance is required, how often is it required, and does the room need to be shut down? Again, shutdowns due to maintenance can impact the cost effectiveness of some room types.
Does the disinfectant damage or degrade materials in the room? Researchers have demonstrated that UV light can damage materials, including fabrics and plastics. A recently published study showed the effect of UV-A light upon irradiated blue fabric swatches in a neonatal intensive care unit 2. Many inks today are cured using UV light and they can fade over time if exposed to UV light of the corresponding wavelength. To date, this effect has not been documented with visible light3.
Finally, the impact of disinfection upon occupants must be carefully considered. Are people comfortable being in a room with UV disinfecting light? Don’t assume so. The public is generally aware of the potential hazard from UV light but often confuses visible light for being UV as well. While visible light can be integrated in a way that makes it appear as typical, white LED light, UV-A light cannot. The study that characterized the effect of UV-A on fabrics2 also documented negative impacts to room occupants — including comments such as “I don’t like the way it [UV] changes the color of everything” and “I really don’t want to be exposed to UV light since I just had eye surgery 13 weeks ago. It sort of hurts my eyes.” The importance of this type of feedback can’t be understated when introducing a new technology to the public. Consensus is needed across a range of groups (users, facilities, medical staff, senior leadership, and so on) to drive proper adoption.
How is system efficacy determined?
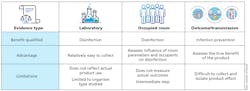
Once the operational parameters of the system — including safety — are established, its efficacy can be determined through one of three ways: laboratory data, occupied room sampling, or outcome or transmission studies.
Laboratory data is the most common method, but it is the easiest to manipulate. It also is not directly translatable to occupied rooms or extended cycles, such as days or weeks. This type of data is more applicable to episodic (noncontinuous) disinfection systems, including UV light and/or chemical disinfectants, such as bleach.
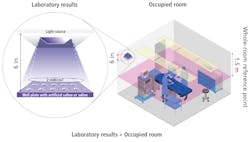
Laboratory data is typically collected at short distances (6 to 10 in.) due to the physical constraints of the biosafety hood. This means that data collected in such a manner can overestimate the performance of the system when compared to its performance in a room setting, as shown in the illustration above.
As explained in my prior linked article, the irradiance at the sample is dependent upon the square of the distance between the sample and the source. For example, in a room with a 9-ft ceiling and a 3-ft task plane, the irradiance could be 144 times less than that used in the laboratory. The effect of this reduction underscores the risk of accepting manufacturer laboratory data in the absence of whole-room data. In an example of laboratory data that is translatable to an occupied room, the Scientific Reports study demonstrating the virucidal efficacy of visible light on SARS-CoV-2 used an experimental setup, where irradiance was reduced to a level found at this 3-ft task plane1.
Occupied room sampling is more difficult to perform than laboratory data because it uses swabbing, impact air samples, or contact petri dishes to recover organisms from the environment. These studies require the accumulation of many samples, taken over a specified period of time, to characterize the effect of the disinfectant in the space. The benefit is that it potentially incorporates the effect people have on environmental contamination, even if the space is between occupied states. Occupied room sampling data is more applicable to continuous disinfection systems, such as visible light and automated , aerosolized hydrogen peroxide systems.
Outcome or transmission studies generate the highest level of evidence and are typically sought by medical professionals. These studies can characterize the effect of the intervention on infections associated with the room or the transmission of organisms within the room. They require advanced scientific techniques, strict scientific rigor, and often a long period of time to statistically quantify effects against other confounding factors.
Outcome or transmission data is the ultimate validation of a manufacturer’s claim and incorporates all the variables — such as dose, time, distance, coverage, and transmission methods — for products that can be confusing or difficult to compare individually. The data also considers the entire room and highlights the ability of a given technology to disinfect the entire space. Studies of this nature are available for portable, episodic UV-C devices and continuous visible-light disinfection4,5.
Microbes matter
Efficacy can further be segmented by organism type, which may present a challenge to individuals without a background in microbiology. Prior to the coronavirus pandemic, users largely classified environmental disinfectant performance against vegetative bacteria (such as Staphylococcus aureus or MRSA) and endospores (such as Clostridium difficile or C.diff). In fact, many environmental disinfection systems have specific output settings for endospores, largely due to their survivability and resistance to disinfectants. UV-C and visible light have demonstrated effectiveness against C.diff endospores6,7, but UV-A has not8. System operators should determine whether efficacy against specific microbes is a concern when evaluating various products. Light sources operated at higher output power, particularly UV LED sources, have significantly reduced source lifetime and are typically only used in unoccupied rooms.
In the face of the COVID-19 pandemic, users representing application environments beyond acute healthcare are now considering whole-room disinfection products. This has highlighted not only the microbiological differences between viruses and bacteria but also the different mechanisms by which these organisms are transmitted (aerosol versus contact with contaminated surfaces). The cited Scientific Reports study showed that visible light can inactivate enveloped viruses, such as SARS-CoV-2 and Influenza-A — previously thought to be unachievable based on earlier measurements against nonenveloped viruses, such as norovirus1. This is an important finding for users looking to improve the safety of room occupants even as the pandemic appears to be waning.
When performed as described, an experimental setup can serve as the basis for more specific claims, in addition to a whole-room claim. Again, this study suggests that visible light disinfection can remove 90% of SARS-CoV-2 within the upper 2 ft of a room in as little as two hours. This is clinically relevant given the cited two- to- three-hour half-life of SARS-CoV-2 in aerosol form. A properly designed and installed visible-light disinfection system can provide protection to room occupants throughout the day while the room is used.
Summary
All forms of disinfection are designed and optimized for a specific set of applications. Potential users of these systems need to be aware of tradeoffs inherent in any design being considered. A whole-room perspective enables users to account for the safety, efficacy, environmental impact, and total cost of ownership in their decision-making approach.
References
1. R. Rathnasinghe et al., “The virucidal effects of 405nm visible light on SARS-CoV-2 and influenza A virus,” Scientific Reports, 11:19470 (2021).
2. J.A. Brons et al., “An assessment of a hybrid lighting system that employs ultraviolet-A for mitigating healthcare-associated infections in a newborn intensive care unit,” Lighting Res Technol, 0:1–18 (2020).
3. D. Irving et al., “A comparison study of the degradative effects and safety implications of UVC and 405nm germicidal light sources for endoscope storage,” Poly Deg Stab, 133:239–254 (2018).
4. D. J. Weber et al., “Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: Focus on clinical trials,” Am J Infect Control, 44:e77–84 (2016).
5. L.J. Murrell et al., “Influence of a visible-light continuous environmental disinfection system on microbial contamination and surgical site infections in an orthopedic operating room,” Am J Infect Control, 47:804–810 (2019).
6. B. Casini et al., “Evaluation of an Ultraviolet C (UVC) Light-Emitting Device for Disinfection of High Touch Surfaces in Hospital Critical Areas,” Int J Environ Res Public Health, 16:3572 (2019).
7. W.A. Rutala et al., “Antimicrobial activity of a continuous visible light disinfection system,” Infect Control Epid,39:1250–1253 (2018).
8. S.H. Livingston et al., “Efficacy of an ultraviolet-A lighting system for continuous decontamination of health care-associated pathogens on surfaces,” Am J Infect Control, 48:337–339 (2020).
Get to know our expert
CLIFFORD J. YAHNKE, Ph.D., chief scientist and head of clinical affairs for Indigo-Clean and Kenall Manufacturing, may be reached at [email protected]. Indigo-Clean is a registered trademark of Kenall Manufacturing Co., a Legrand company. Kenall was founded in Chicago in 1963 and has built a reputation for durable lighting solutions. Today, the company creates solutions for the healthcare, cleanroom/containment, food processing, transportation, high abuse, and correctional lighting markets. Kenall luminaires are designed in Kenosha, Wisconsin, and comply with the Buy American Act (manufactured in the United States with more than 50% of the component cost of US origin). For additional information, visit indigo-clean.com.
Kenall manufactures and supplies 405-nm disinfection technology, which was utilized in the study cited as reference 1.
Abridged version of this article was published in the April/May 2022 issue of LEDs Magazine.
For up-to-the-minute LED and SSL updates, why not follow us on Twitter? You’ll find curated content and commentary, as well as information on industry events, webcasts, and surveys on our LinkedIn Company Page and our Facebook page.