Myopia is now regarded by the World Health Organization (WHO) as a global epidemic. On average, developed and developing nations have experienced a three-fold increase in myopia over the past three decades. There is debate as to the reasons for the increase, but there appears to be correlation with the increase and the fact that many children today don’t get sufficient exposure to natural light (Fig. 1). Solid-state lighting (SSL), with tunable spectral power distribution (SPD) enabled by LED sources, however, may have the ability to change the trend by offering exposure to specific wavelengths needed for normal development of the eye and emmetropia or no visual error.
Stepping back, let’s review some facts on myopia or nearsightedness. In South Korea, China, and most of eastern Asia, 80% of high school graduates and 95% of university graduates are myopic. Apart from the personal costs, inconvenience, and disability, myopia brings elevated risk of ocular morbidity for many of these sufferers in later life such as glaucoma, macular degeneration, retinal detachment, and cataracts, which in turn poses the risk of an onerous burden on health and welfare costs both at a personal and state or governmental level.
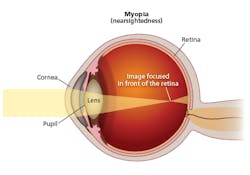
Myopia is a disorder where light passing through the cornea, pupil, and lens focuses at a point in front of the retina rather than at a point right on the retina (Fig. 2). There are multiple potential causes of the mismatch in focus. But one cause is abnormal eye growth or elongation in children.
Myopic causes
Rising incidences of myopia were once thought to be due to prolonged reading and close work. Other researchers have studied the possibility that genetics led to the increase in myopia rates. Researchers in the vision sciences, however, have shown that a leading cause of myopia is reduced exposure to specific wavelengths in sunlight and their beneficial effects beginning at the retinal level.
Indeed, laboratory research and studies on trends in myopia within human populations now suggests that the “retinal dopamine response” — the release of the dopamine chemical that acts as a neurotransmitter, instigated by the non-visual light receptors of the retina — controls eye growth and therefore the pathogenesis of myopia. The remedy for myopia is to be found in strategically beneficial wavelengths of sunlight.
It turns out that the issue of increased myopia is closely related to the same non-visual elements of eye physiology that have been tied to other maladies such as circadian disruption. Researchers have revealed that non-visual neural elements found within the innermost layer of the retina, the intrinsically photosensitive retinal ganglion cells (ipRGCs), are central in contributing to the natural circadian rhythm. Light stimulus of ipRGCs therefore is considered the “primary zeitgeber” for the human circadian/physiological cycle. See LEDs Magazine coverage of the Lighting for Health and Wellbeing Conference for more information on the non-visual system.
FIG. 2. Myopia results when light through the front of the eye is focused at a point in front of the retina.
Similarly, this article examines the neuro-physiology of the visual and non-visual processes responsible for achieving emmetropia. and how with current LED technology the strategic application of appropriately designed human-visuo-centric tunable LED lighting can play a vital and essential role in supporting the eyesight of humans in a way that minimizes the incidence and progression of myopia while also essentially supporting the natural circadian cycle.
Growing global incidence
As noted earlier, myopia is clearly on the rise. Research published by the Brien Holden Vision Research Institute at the University of New South Wales, Australia in 2016 revealed the alarming statistic that at current rates, by 2050 half the world’s population will be myopic. Research has documented the trend that is pronounced in parts of Asia where children are tasked with many more hours of daily homework than are their counterparts in the US, Europe, and other regions. And the culprit isn’t the lengthy study period but the lack of outdoor activity.
Researchers have cemented the relationship between lack of daylight exposure and increased myopia. Australian researchers and professors Kathryn Rose and Ian Morgan compared Chinese school children aged 7 years in Sydney with those of the same age and ethnicity in Singapore; the incidence of myopia was about 10× greater in those children in Singapore where they were exposed to 75% less sunlight on average per week. The question raised was: “What is the essence within sunlight that provided this benefit?”
Tracing the cause
Back to the ipRGCs, preservation of the circadian rhythm and the pupillary light reflex in certain eye pathologies made researchers long consider the existence of a mechanism that contributed to our circadian rhythm and pupillary light reflex quite apart from the rods and cones that underlie human vision. Researchers had discovered that numerous subjects without light perception still experienced circadian entrainment. This suggested a retinal system sensitive to light but not projecting to the visual cortex — rather to an area within the mid-brain controlling the release of melatonin, the supra-chiasmic nucleus (SCN).
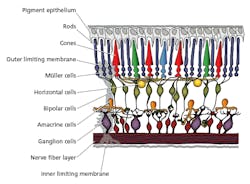
The ipRGCs were found to produce melanopsin as the photo-pigment within ipRGCs that governs the response to light, contributing significantly to the brain’s influence over the circadian rhythm and also the sustained pupillary light reflex. Meanwhile, the primary source of retinal dopamine is found in the amacrine cells (Fig. 3); their cell bodies reside in the inner nuclear layer of the retina and synapse directly with ipRGCs found in the inner-most ganglion cell layer of the retina. The melanopsin photopigment found in ipRGCs responds maximally at 480 nm; so maximal release of retinal dopamine must be elicited by stimulating the ipRGC/amacrine cell pathway by exposing the retina to an adequate stimulus of light rich in energy at 480 nm. This corresponds with the wavelength that triggers maximum inhibition of melatonin from the pineal gland.
FIG. 3. The human retina is sometimes referred to as inverted and light first reaches the ganglion cells before the rods and cones that play a key role in forming an image. The amacrine cells are primarily responsible for retinal dopamine.
The ipRGCs therefore perform a dual function as the primary receptors for management of circadian entrainment and also are central to the emmetropization process regulating eye growth. The ipRGC response has a two-fold effect: first, a neurological and intrinsic response to light by the depolarization of the cell by photon transduction of melanopsin that is directed to the mid-brain via the optic tract to the SCN within the hypothalamus; second, by synaptic communication with retinal amacrine cells, the release of dopamine that influences eye growth.
Neuro-anatomy of the retinal dopamine pathway
Several classes of ipRGCs have been identified, namely M1 to M5. M1 ipRGCs have a strong intrinsic photo-response and project first to the SCN for circadian control and second to the olivary pretectal nucleus (OCN) accommodating the nucleus of the third cranial nerve controlling pupillary response. M1 ipRGCs are therefore responsible for the sustained pupillary constriction with their fibers projecting to the nucleus of the third cranial nerve (in the area of the superior colliculus) then returning anteriorly ultimately to the sphincter pupillae muscle within the iris. Retinal light stimulus by this pathway reduces the pupillary area in brighter light, thereby autonomically limiting retinal illuminance.
As light passes through the retina and stimulates ipRGC response particularly with light rich in blue at 480 nm, the response of ipRGCs is seen at various levels. There will be inhibition of the release of melatonin from the pituitary gland, thereby supporting a wakefulness period, and there will be stimulation of amacrine cells to release dopamine. As will become relevant later, it should be noted that pharmacologically, melatonin and dopamine are antagonists. A sustained period of exposure to light and particularly blue light at 480 nm causes a sustained firing of the ipRGC/amacrine cell pathway and consequent release of retinal dopamine.
Other researchers further studied the mechanism behind retinal dopamine and its effect on eye growth. There is interaction of dual systems of retinal dopamine receptors, respectively D1 and D2. Balanced stimulus of the two results in emmetropia. Researcher Scott Read and colleagues showed that approximately 840–1450 lx in the 480-nm band had a positive effect on reducing axial elongation of the eye. Hua and colleagues showed increasing the available luminance at desk height of school children to 500 lx had a positive benefit in reducing the incidence of myopia.
Collectively, the research indicates an opportunity for tunable LED lighting that could provide the necessary wavelengths and luminance over time for required retinal responses to positively impact eye health, reducing the frequency and progression of myopia. A solution would depend on providing adequate average daily lux of adequate duration with the correct SPD to trigger the retinal dopamine response while also respecting the circadian rhythm.
It is important at this stage to note other work by Ayaki in 2016 that revealed children with poor sleep quality were more susceptible to high myopia. This link between the status of eye growth and a history of sleep disruption points strongly to the link between satisfactory retinal illumination which in turn provides support and respect of the natural circadian cycle. Any solution that sets out to address the retinal dopamine response should also take into consideration the over-arching importance of supporting the natural diurnal circadian rhythm.
Design of the tunable prototype
Let’s consider a prototype LED system that might lessen myopia rates that was built and tested. The SPD of a suitable LED luminaire must be tunable, emit white light, and retain a blue peak at 480 nm, offering maximal opportunity to stimulate ipRGC/amacrine cell release of retinal dopamine in concert with preferably the diurnal cycle of the human experience of sunrise to sunset. Further, the blue peak of 480 nm should ideally be higher in energy in the morning and be preferably reduced over the period of a day’s exposure more or less linearly so that by late afternoon there is minimal radiation at the wavelength to which ipRGCs are most sensitive. While the governing parameters in this solution are based on wavelength and luminance over time, it is for convenience reasonable to describe here in broad terms that this solution would display a higher correlated color temperature (CCT) of around 5000K in the morning and a reduced CCT of around 2700K in the afternoon.
There would be a number of safety and logistical issues in developing a tunable SSL system to provide the desired response. For instance, such a system must be designed safety cognizant of the potential risk from the blue-light-hazard and exposing the retina to wavelengths in the range of high-energy visible light (HEV) in the 415–455-nm range for any extended period. The proposed system recognizes this and has design elements that observe these risks and complies with current standards, including ANSI/IESNA RP-27 and IEC/EN 62471 2008. It is noteworthy that while sunlight of course contains wavelengths of 415–455 nm that researchers have identified as potentially hazardous to the retina, it is also rich in energy at 480 nm, which ensures maximal sustained pupillary constriction by virtue of the ipRGC response described earlier.
But typical phosphor-converted white LEDs typically have a trough in the SPD at 480 nm, so maximal pupillary constriction may not be afforded the eye to autonomically provide its own natural protection against the shorter blue wavelengths. Consideration must be given also to the greater transparency of younger eyes and scenarios where the proposed system may be deployed (kindergartens, schools, and offices). It is essential that protection of the retina from HEV is made a priority in this solution, particularly when offered as a therapeutic benefit.
Equally important, any light source with a blue peak at 480 nm should be tunable in order that the SPD and luminance can be altered to respect the natural systemic circadian rhythm. The blue energy should decrease over time, essentially following the natural changes in the sun’s spectrum consistent with the time between sunrise and sunset.
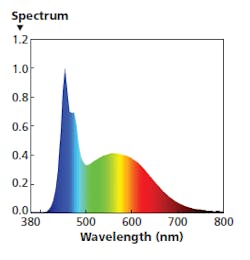
FIG. 4. An SPD with a blue energy peak extended to 480 nm is ideal in the morning both to entice circadian wakefulness and to stimulate the dopamine response that encourages optimal eye growth in children.
Luminaire development options
Moving to LED selection and luminaire design, there are a number of limitations relative to LEDs widely used in general lighting in terms of generating the optimum SPD that would encourage emmetropia. LED sources with a blue pump and phosphor coating, by virtue of the dipole effect of the blue photons causing excitation of the yellow phosphor, produce white light with a blue peak within the potential blue-light-hazard range for human eyes at 440–450 nm. The challenge is the production of a tunable SSL systems that has a blue peak at 480 nm, which will still render light white to the human eye with a comparable CRI to current LED technology with the blue peak shifted from the hazardous range.
When deployed to act as lighting to prevent myopia, the cardinal variables of wavelength, luminance, and time should be strategically adjusted in parallel with the diurnal solar cycle to entrain and support the circadian rhythm. The elevated peak of 480 nm is optimal for morning reducing linearly during the day with a sum of light exposure approaching an average of 14,000–15,000 lux-hours per week. Such output ensures appropriate stimulus of both retinal dopamine response and entrainment of the natural circadian rhythm.
The prototype system was a relatively simple design intended to allow accurate measurement of the SPD and to observe the nature of the light produced. The luminaire was designed using three modular LED light engines with different SPDs. A computer can control each of the three channels via software. One channel/light engine produces monochromatic 480-nm energy. Then the system includes warm- and cool-CCT light engines, enabling a broad mix of SPD output via dimming of the three channels. The prototype system was tested by an independent laboratory to characterize the SPDs detailed in Figs. 4, 5, and 6. And clearly the output could be tuned to points in between.
Testing the design concept
The SPD in Fig. 4 is intended for morning usage. The cool-white LED light engine is operated at 100% output. The 480-nm engine is also operated at a lower level, extending the width of the blue band. The warm-white engine operates at a very low level, limiting energy in the red range. The combination delivers 700 lx at desk height with a CRI Ra around 88 and CCT near 8000K, ideal for the 9:00–11:00 AM timeframe in, say, a school setting.
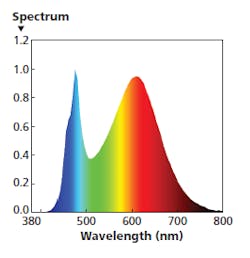
FIG. 5. Near midday, an ideal SPD would have a far greater level of red energy and a significantly warmer CCT.
For the 11:00 AM to 1:00 PM time period, you might use the SPD shown in Fig. 5. In that example, the warm-white light engine is operating at 100% while the other two engines operate a much lower level such as 10%. That mixture has delivered red energy at a magnitude that approaches the blue peak. The recommended settings deliver 550 lx at desk height with a CRI around 83 and CCT around 3300K.
Moving to needs for later in the day, the SPD in Fig. 6 illustrates an even more dramatic change. In that example, the warm-white engine is operating solo at 100% with the other two engines at 0% output. The mix delivers 350 lx, CCT around 2600K, and CRI around 83. The setting would be recommended for the 1:00–4:00 PM time range.
FIG. 6. In the afternoon, an ideal SPD is almost devoid of blue energy with the eye having produced sufficient dopamine and the circadian system beginning the transition toward evening.
The prototype tunable LED lighting system had relatively simple controls. But a fully integrated computer-controlled system could slowly vary the output on a continuous basis throughout the day.
Conclusion
The growth of the human eye is a perfectly normal event from the age of two years (rapid eye growth) to nine (slow eye growth). By the age of nine years, the human eye should have reached emmetropia and normalization of axial length. Under normal day/night exposure and provided with adequate sunlight rich in indigo-blue wavelengths of around 480 nm for at least a part of the solar day, the eyes will naturally maintain a state of emmetropia, meaning that no optical correction will be necessary to render an in-focus retinal image. It is a complex process and quite remarkable given that that there is wide variation in the optical properties, particularly focal lengths, of the cornea and crystalline lens that require precise adjustment of eye length to achieve a clear retinal image.
Not uncommonly, corneas with a central radius that vary significantly from the human average of 7.8 mm are to be found associated with an eye with little or no refractive error. This occurs despite the fact that the cornea contributes around 44 Diopters out of a total power of 60 Diopters of the total power of the average eye — a variation of only 2–3% of corneal power would arithmetically vary the refractive power of the eye by about 1 Diopter. However, clinicians routinely measure wide variations in corneal power among the cohort of their patients without seeing a commensurate association with visual error. The eye and brain have cooperatively matched corneal power and axial length to render the eye emmetropic. This represents the power of the emmetropization process that can be harnessed by appropriate exposure to requisite light wavelengths and luminance levels.
There are currently various methods available to effectively intervene in the attempt to reduce progression of myopia. Recent research has highlighted the use of low-dose atropine eye drops. In the case of atropine, the side effects of blurred vision and a heightened awareness of glare notwithstanding, the benefit is typically lost when the atropine drops are stopped. The most efficacious treatment using low-dose atropine showed a 2.00-Diopter increase in myopia one year after ceasing the treatment.
In the hands of the author, the prescribing of multifocal spectacles and, historically before that, bifocal spectacles, helped to slow the progression of myopia in the eyes of younger patients most often followed for the period of their schooling and sometimes beyond. These approaches have some merit; however, they are an attempt to remedy the problem after its onset. These interventions are also costly, and in the case of atropine eye drops, not without side effects.
ipRGCs play multiple roles
The initial pupillary response to a visual stimulus is generated by the response of the visual sensory elements, rods, and cones. Within a second or less of this initial response however, the ipRGCs fire their action potentials to then maintain pupil size (the pupil being slightly more dilated than with the initial pupillary rod/cone response), subject to retinal illuminance. Providing the ipRGCs with the optimal wavelength of 480 nm for absorption by their own retinal opsin, melanopsin, ensures maximum pupillary constriction for a given retinal illuminance. Consequently, the ipRGC pupillary pathway that maintains a smaller pupil provides optimal optical conditions which are the most ideal for an in-focus retinal image and minimal higher-order aberrations of the retinal image, shown elsewhere to be positively associated with progression in myopia.
Recent research also shows a strong association between elevated morning concentrations of melatonin in myopic adults compared to non-myopic adults. If we conclude from Read’s research that myopic subjects are subject to less light exposure, it is an interesting connection to relate this to the elevated melatonin found in myopic subjects. As melatonin inhibits dopamine, there is a reasonable case to make that elevated serum melatonin is inhibiting the activity of retinal dopamine essential for normal eye growth.
While there is strong evidence for the local beneficial retinal/ocular effects of dopamine contributing to eyes maintaining emmetropia, it is logical given the research to date that support of the systemic circadian rhythm with a phased variation in wavelength and luminance over time is another important component of the solution. Photo-entrainment of the non-visual eye-brain processes serves the dual purpose of entraining both the retinal and systemic circadian cycles. At both the local level and at the systemic level, the primary zeitgeber for photo-entrainment (light) in this solution is provided at an optimally prescribed formula and dose subject to time of the solar day.
The prior research of Read and Hua revealed that once an adequate dose of light was provided, myopia was lessened in both progression (Read) and incidence (Hua). The work of Hua was outstanding as it showed that simply increasing room illumination offered a remedy in the context of prior research targeting the beneficial effects of sunlight. Developed in a tunable system configuration with an optimized SPD, LED illumination offers an idealized solution that can be prescribed to respect, support, and entrain the human circadian rhythm.
The exhaustive work over many years by laboratory researchers revealed the vital role of the retinal dopamine response in controlling eye growth. Unraveling the neurobiology and neuro-anatomy of this system over time has helped greatly explain the laboratory results, field results, and epidemiology of myopia.
DR. STEPHEN MASON is an optometrist having earned a bachelor of optometry and is a Fellow of the American Academy of Optometry (FAAO). He is also founder and managing director of Sustainable Eye Health Pty Ltd., based in Australia, and has presented at LEDs Magazine’s Lighting for Health and Wellbeing Conference.